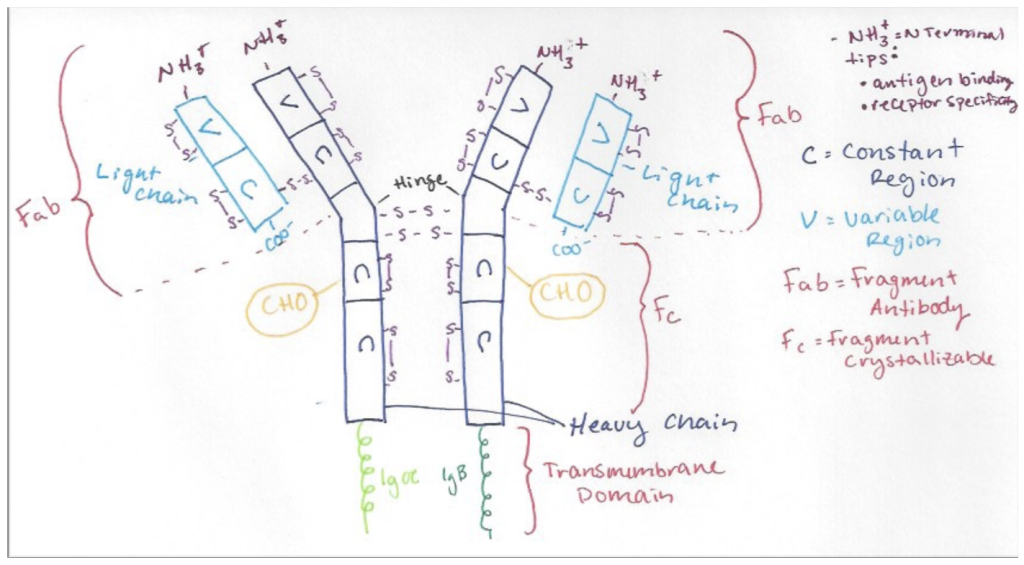
IgM, as drawn above, is a quaternary protein consisting of two identical heavy chains and two identical light chains. Within each chain are variable and constant regions. The Fragment-antibody region includes the heavy and light chain, and the N-terminal tips. The N-terminal tips are involved in antigen binding and give the receptor its specificity and the variability of the heavy/light chain beta-sheet loops is higher near these tips. Disulfide bonds are largely responsible for maintaining the structure of IgM and can be seen above in purple. In the crystallizable fragment region, the CHO label demonstrates where glycosylation occurs in the rough ER and Golgi apparatus before adhering to the cell surface. IgM has 14 glycosylation sites (only two pictured). When IgM is soluble, it acts as an antibody and is secreted as a pentamer. The pentamer is also held together by disulfide bonds, and an additional J-chain that the membrane bound structure lacks. When the structure is membrane bound, it presents as a monomer with a transmembrane domain. The Ig alpha and beta are signal transducing subunits. These subunits have one heavy chain locked between them to comprise a juxtamembrane region (not demonstrated above).